






Researchers should be aware of and comply with any procedures and guidelines provided by their institution, including procedures for collecting and recording data generated from their research, see Melbourne Health: CRFs, Source Documents, Record Keeping and Archiving, as an example.
Data used in clinical trials should always be collected from the source. That is the original location where the data was captured.
The term source data is used to describe all information in original records and certified copies of original records of clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the trial.
Source data are contained in source documents, the original documents, data or record. These include:
The trial Data Management Plan should describe all source documents that will be used to collect data for the trial.
It is important to make clear to all study team members, study monitors (where applicable) and potential auditors which document is the source document for each data item.
The source data and their capture methods should be clearly defined prior to participant recruitment, ie in the protocol or in a study-specific source data plan.
The resources contain two examples of a source document plan/log that are available for download.
This list should be prepared by the site and signed and dated by the principal investigator. The list should be filed in the Investigator Site File.
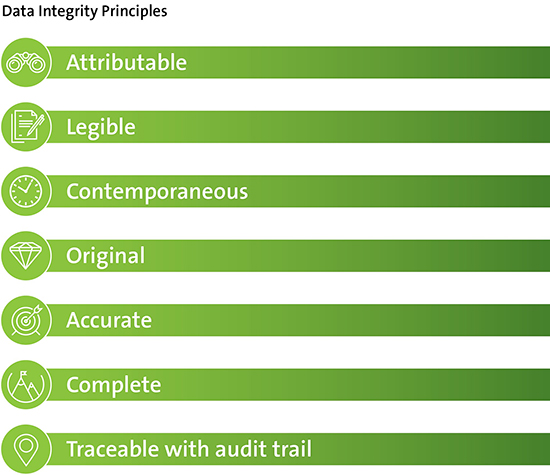
The Principal Investigator and institution both have responsibilities for maintaining adequate and accurate source documents and trial records that include all pertinent observations on each of the site’s trial participants. The source data should be appropriately managed to ensure they are:
Data management begins with collecting your data. Each trial will require a data collection tool called the case report form (CRF) to record all of the protocol required information to be reported to the sponsor on each research/trial participant. The CRF may be paper or electronic.
In accordance with Australian privacy laws, everyone has a right to have their personal information kept private.
Researchers have responsibilities for managing their clinical trial data under the Privacy Act 1988 (Cth) (Privacy Act) and the applicable State and Territory legislation. For example in Victoria, the Health Records Act 2001 (Vic) (Health Records Act) applies.
The Privacy Act (1988), including the Australian Privacy Principles (APPs), and the Victorian Health Records Act, including the Health Privacy Principles (HPPs), regulate the collection, use, disclosure and handling of personal information.
Researchers should be familiar with the requirements of all applicable privacy legislation and the privacy policy of their institution.
In accordance with the NHMRC National Statement on Ethical Conduct in Human Research, unless there is justification not to do so, researchers must adopt methods to reduce the risk of identification of participants during the collection, analysis and storage of data and information.
Steps to reduce identification of participants include:
Limit participant identifying data to what is necessary to address the study aims. The more identifiers that are collected, the increased potential to re-identify an individual.
Tag fields containing participant-identifying data and restrict access.
Manage user access throughout the life cycle of the trial (eg that site-specific access is implemented where appropriate; that expired or suspended user accounts are removed).
Also consider if there are other global requirements for data collection and privacy that are relevant to the trial. For example, researchers collecting data from participants based in the EU will need to comply with the General Data Protection Regulation (GDPR).
Note: In accordance with the National Statement, it is not recommended to use the terms ‘identifiable’, ‘potentially identifiable’, ‘re-identifiable’, ‘non-identifiable’ or ‘de-identified’ as descriptive categories for data or information due to ambiguities in their meanings. Re-identification and de-identification are best understood as processes that change the character of information and are only used with this meaning.
