






Data management begins with collecting your data. Each trial will require a data collection tool called the case report form (CRF) to record all of the protocol required information to be reported to the sponsor on each research/trial participant. The CRF may be paper or electronic.
Electronic web-based CRFs are commonly used. These systems may also be used to build the trial database for processing and storing the data during the study.
Researchers should enquire within their own organisation as to what data management software packages they have available. REDCap is commonly used but other systems may be available, such as OpenClinica.
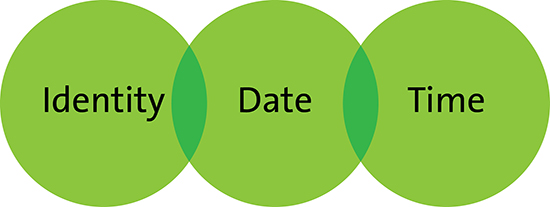
At a minimum, all trials require a data management software system that provides an audit trail of all data entered including:
Note, Microsoft Excel does not provide an audit trail and should not be used to manage clinical trial data.
The CRF design should capture all the data required by the protocol – no more, no less. Capturing more data is not ethical and can be detrimental to your study by placing additional burden on participants and staff. Not capturing all the data specified in the protocol can jeopardise the data analyses and the success of your trial.
Researchers should have access to training and/or guidance materials within their institution to assist with designing the CRF and trial database for their trial. This may also be supported by training videos and help pages available through the chosen platform, such as REDCap or OpenClinica.
Before the CRF is finalised, members of the trial team should have input into the design and review and this should be evidenced by formal approval. The review team should include the Sponsor-Investigator, study coordinator(s), statistician and members of the data management team.
The CRF must be finalised before the first participant is recruited. As the CRF development takes time, consider beginning the process during the protocol writing phase.
To support consistent, accurate and complete collection and recording of data, site staff and monitors require training on how to complete the CRF. This requires trial-specific CRF Completion Guidelines, either as a stand-alone document or as part of a study manual of procedures.
The Sponsor-Investigator is responsible for developing the CRF Completion Guidelines and ensuring that all trial staff involved with collecting, entering and reviewing data in the CRF have received training in these guidelines, before they start their delegated task for the trial.
Best practice guidance on the development, maintenance, and implementation of these guidelines is available in the Society for Clinical Data Management (SCDM) GCDMP Chapters: CRF Completion Guidelines.
Recommendations in this guideline are in accordance with the requirements:
All users must be given a unique username and password to gain access to the CRF and trial database based on roles/permissions delegated by the Principal Investigator. Permissions include data entry or data view only. This level of permission should be evident in the study Delegation of Duties log.
Paper records should be stored securely in a locked location.
Permission to access data (both during and after the study) must also be in accordance with the conditions of consent provided by participants.
