






The Australian Teletrial Program (2022) provides the following definition:
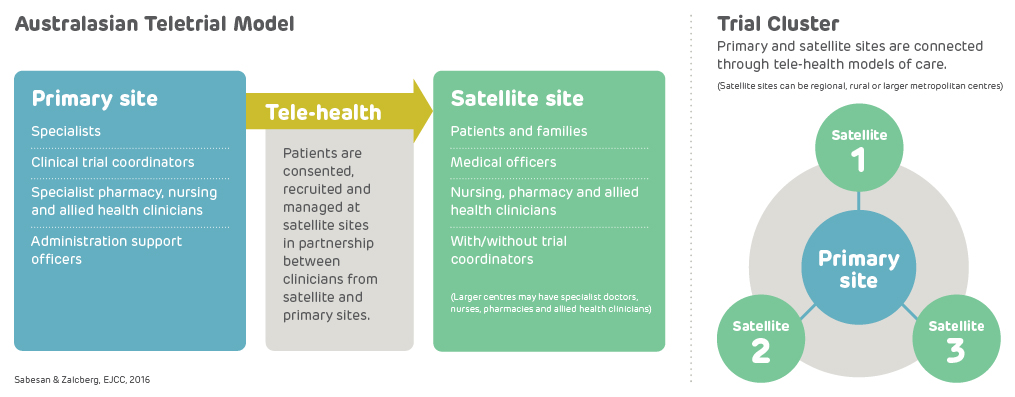
A teletrial is a group of clinical trial sites that work together to conduct a clinical trial under the supervision of a Primary Site.
A teletrial uses telehealth technology to communicate between the Primary Site and Satellite Site/s and enables delivery of aspects of a clinical trial closer to home for patients, particularly in regional, rural and remote locations. A Principal Investigator supervises Associate Investigator/s to conduct a clinical trial at a Satellite Site which is geographically remote from the Principal Investigator’s Primary Site.
The Principal Investigator remains responsible for the trial. A detailed Supervision Plan is required, in addition to a Delegation Log required by ICH GCP for all Satellite Sites regardless of experience. Trial participants may have trial visits at both the Primary and Satellite Sites, as determined by the Protocol and Supervision Plan.
The term Decentralized Clinical Trial (DCT) is often used by sponsors as an umbrella term. Teletrials are a 'sub-component' or a type of DCT.
This idea and model do not specify the difference between a Primary and Satellite Site but conceive of a spectrum of activities that may be interpreted according to sponsors' and clinicians' needs. Essentially very similar to Teletrials but there are not enough published examples yet to determine fixed models.
McKinsey & Company Decentralized Trials, Article 2021
Position Paper on decentralised clinical trials (DCTs) with medicinal products in Switzerland
A fully virtual clinical trial is one that is completely technology-based and can include those run by registry searches and data mining. That means there are no traditional sites, no physical locations used, and no face-to-face interactions. Actual participants may be involved but all interaction is via websites, phones, apps, watches, and e-diaries.
https://www.cosa.org.au/media/hallsjb3/cosa-teletrial-model-final-19sep16.pdf
National teletrials compendium: https://www.health.gov.au/resources/collections/the-national-teletrials-compendium
Queensland Health teletrials information page: https://www.health.qld.gov.au/hiiro/html/teletrials
Medicines Australia Teletrials Resource page: https://www.medicinesaustralia.com.au/policy/clinical-trials/tele-trials/
