






New approaches to cancer clinical trials aim to support rapid, cost-effective studies that effectively answer clinical questions to improve patient care.
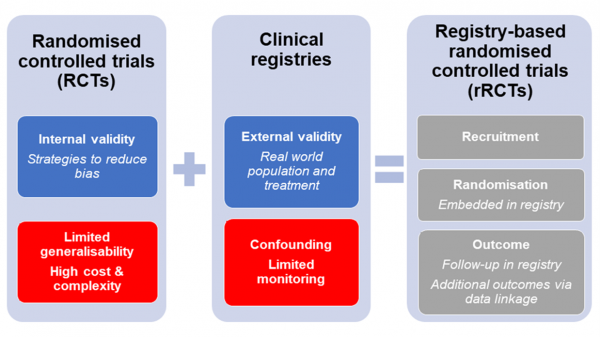
The Registry-Based Trials Program combines conventional trial methodology with registry systems to produce real-world clinical evidence. Registry-based trials integrate the high internal validity (elimination of bias) of randomised clinical trials with the high external validity (applicable to a clinical setting) associated with enrolling real-world patients through a registry.
We've developed an free online training program for clinical trial professionals to learn more about how to conduct registry-based clinical trials. Click below to get started.
Randomised-controlled trials (RCTs) are a traditional clinical trial method that provides high-level scientific evidence and is the gold standard for comparing care approaches or treatment options. RCTs facilitate rigorous evidence to support clinician decision-making. However, RCTs commonly have narrow eligibility criteria, limiting patient recruitment and external validity, as well as substantial per patient costs.
Clinical registries are a database that can be designed to be disease, health services or product specific. They collect clinical information for a specific area of interest, and depending on the depth of clinical detail captured, can support a variety of research questions. Longitudinal follow up within a registry, as well as potential data linkage to other routinely collected data software, e.g. electronic health records or National Death Index, allows for sound representation of real-world patients. However, registries are limited as their output can report associations and only hypothesise causality.
When a randomised controlled trial is embedded within a registry, it is known as a registry-based randomised controlled trial, or registry trial (rRCT). Optional add-on trial modules are developed to allow for randomisation and specific trial data (e.g. patient reported outcomes), with data collection achieved via the registries during routine care practices, delivering valuable infrastructure for collecting baseline and outcome treatment data. This allows rRCTs to operate with a broad eligibility criteria, enabling trial access to a large group of participants who are reflective of real-world patients. This innovative, yet pragmatic trial design allows for rapid recruitment and at a significantly lower cost than a conventional randomised controlled trial.
The registry based trials approach has demonstrated excellent results in international cardiovascular trials, generating high impact publications that have changed standard of care treatments. The VCCC Alliance Registry Trials Program have helped to expand this approach in an oncology setting, with actively recruiting trials such as ALT-TRACC, EX-TEM, REAL-PRO and AVATAR.
Registry based trials, in this program, are conducted on specific tumour streams including both common and rare cancer types. Data is collected in routine care processes from a range of health services, enabling recruitment of a large patient group. In-depth methodology and design analysis is undertaken to provide a critical evaluation of the registry trials approach and to identify success factors.
Download Steps to setting up a Registry Based Trial
Hypothetically, the registry based trials approach is a suitable, cost-effective way to pursue clinical oncology questions, while maintaining trial rigour. An Australian survey of exploring perspectives of clinicians and clinical trial unit coordinators conducting registry based trials have reported that the approach is ‘simple and straightforward’ and maintains clinical equipoise when compared to traditional commercially sponsored clinical trials.
Registry trials can be conducted within usual care and are a means of introducing early career clinicians to initiating and leading clinical trials; increasing investigator experience.
VCCC Alliance partners are a critical mass of proven clinical trial facilities and infrastructure. Partner clinical sites, as well as other health services, provide resources to promote trials, support recruitment and deliver data management and analysis.
A research fellow from the Evaluation and Implementation Science Unit, Centre for Health Policy, University of Melbourne is conducting the evaluation. Meticulous analysis and evaluation of oncology registry trials will provide beneficial insights for local and international health services as well has community health outcomes.
A comparison of conventional clinical trials and registry-based randomised controlled trials in multidisciplinary cancer care:
Foroughi S, Wong HL, Gately L, Lee M, Simons K, Tie J, Burgess AW, Gibbs P. Re-inventing the randomized controlled trial in medical oncology: The registry-based trial. Asia Pac J Clin Oncol. 2018 Jun 26. doi: 10.1111/ajco.12992.
Defining key design elements of registry-based randomised controlled trials: a scoping review
Karanatsios, B., Prang, KH., Verbunt, E, Yeung JM, Kelaher M & Gibbs P. Defining key design elements of registry-based randomised controlled trials: a scoping review. Trials 21, 552 (2020). https://doi.org/10.1186/s13063-020-04459-z
Professor Peter Gibbs, Clinical Program Lead and Medical Oncologist
Dr Vanessa Wong, Clinical Research Fellow and Medical Oncologist
For further information about the VCCC Alliance Registry Trials program, contact Duncan Colyer, Program Manager: Clinical Trials Innovation ([email protected]).
