






It is important to have support from a Biostatistician in the planning stage of the trial.
Key activities that require input from a Biostatistician
Many institutions have in-house Biostatisticians who can provide statistical support at no cost for researchers for a defined number of hours per project. It is important to consider that complex trial designs may require additional support and to consider this cost when working out the budget for the trial.
This activity takes time and care to ensure the data collected for the trial is complete, see Data Management.
In accordance with GCP, the sponsor is responsible for implementing and maintaining quality assurance and quality control systems with written standard operating procedures (SOPs) to ensure that trials are conducted and data are generated, documented (recorded), and reported in compliance with the protocol, GCP, and the applicable regulatory requirement(s).
Some of these SOPs may be generated by the sponsoring institution but others will be the responsibility of the Sponsor-Investigator.
The Sponsor-Investigator is responsible for developing any SOPs that are required to ensure compliance with the trial protocol. Examples of tasks that may require an SOP include:
Institutions should have an SOP template that researchers can use that will assist them to include all the necessary elements.
Depending on a person’s role in the trial, there will be different training requirements specific for the trial. Both the sponsoring institution and the Sponsor-Investigator should establish the appropriate training requirements for each role in the central coordinating trial team and participating sites clinical trial team(s).
The sponsoring institution should have a policy regarding who should undertake GCP training.
For example, Principal Investigators and Study Coordinators should all complete a course that meets the TransCelerate Biopharma Minimum Criteria for ICH E6 CP Investigator Site Personnel Training.
For further guidance, refer to the National Clinical Trials Governance Framework and User Guide.
For other staff, it may be appropriate to receive GCP training that is tailored to their role and responsibility in the trial. For example emergency department doctors or nurses who are only responsible for obtaining informed consent from participants may not require full GCP training.
The Sponsor-Investigator and Site Principal Investigator have responsibilities for ensuring their staff receive any training required by their institution relevant to their role, in addition to any trial-specific training, such as administration of investigational product or complex trial assessments.
Training documentation, including GCP certification, attendance at courses, reading SOPs, must be maintained and retained for all members of the clinical trial team. Records are maintained in the Trial Master File (for Sponsor-Investigators and the central coordinating trial team) or Investigator Site File (for the Site Principal Investigator and site staff).
Important information that researchers can obtain when consumers are involved in the study design includes:
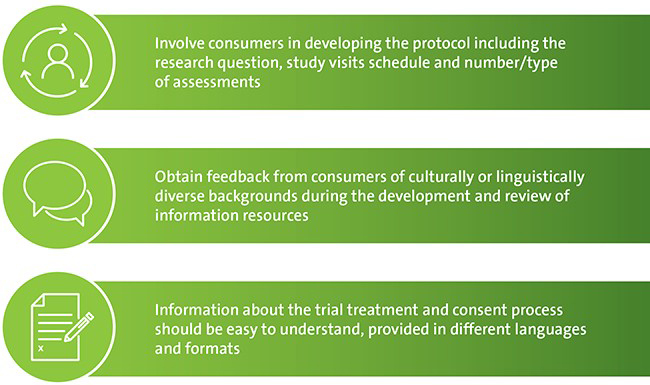
Partnering with consumers is one of the five components of the National Clinical Trials Governance Framework. This new framework lists actions that a health service organisation must meet if it provides a clinical trial service.
Researchers should be familiar with and follow their institution’s policies and processes for partnering with consumers, or see the VCCC Consumer Engagement toolkit and resources.
