






The ethics submission process for a telehealth trial is similar to that of a traditional trial but requires a few additions. There are many roles who need to come together to ensure the timely and successful submission of HREC and RGO submission for a teletrial. These include the Primary Site HREC, Primary Site RGO and the Satellite Site RGO. This page outlines a step-to-step guide through the HREC and RGO submission process.
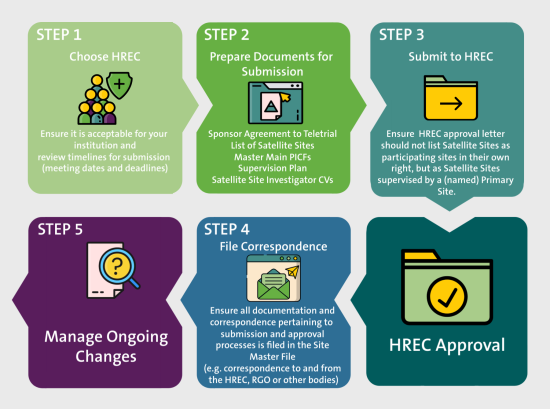
Prior to HREC submission, the investigator must do the following:
Ensure all teletrial-specific documents are prepared before submission along with the documents needed for a traditional trial.
Use this HREC submission teletrial documents checklist to ensure all required teletrial-specific documents are included:
Submit the ethics application through the relevant reviewing HREC submission process.
Ensure all documentation and correspondence from the submission and approval is filed in the Site Master File.
HREC must be notified of any changes after HREC submission such as changing a standard approved clinical trial to a teletrial or adding additional Satellite Sites to an approved cluster already using the teletrial model. An amendment may be needed.
The Primary Site takes responsibility for all reporting to the CPI on behalf of the satellite sites. This includes ongoing reporting to HREC, annual reports and safety reports.
Primary Site Teletrial Documents checklist for PS RGO Submission:
Satellite Site Teletrial Documents checklist for SS RGO:
